Simulation ionic and covalent bonding answer key – Embarking on a scientific odyssey, we delve into the captivating realm of simulation ionic and covalent bonding, unraveling the intricate details of these fundamental chemical interactions. This comprehensive exploration, guided by an authoritative answer key, empowers us to decipher the complexities of molecular formation and unravel the secrets of chemical bonding.
As we traverse this intellectual landscape, we will dissect the fundamental concepts of ionic and covalent bonding, examining the electron transfer and electron sharing mechanisms that govern their formation. Furthermore, we will meticulously compare their distinctive properties, deciphering the implications of these differences for the compounds they form.
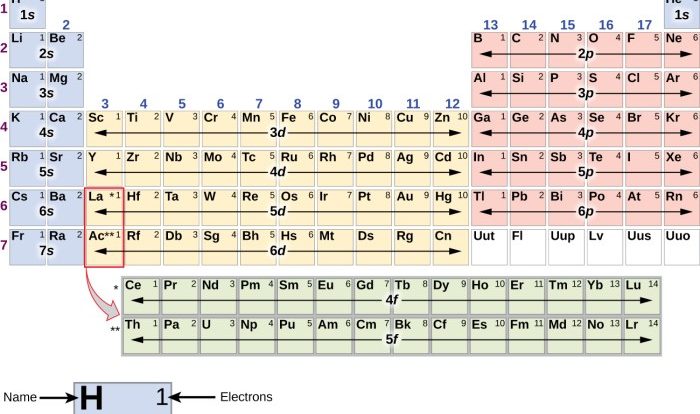
Ionic and Covalent Bonding
Chemical bonding is the process by which atoms and molecules interact with each other to form stable structures. There are two main types of chemical bonding: ionic and covalent.
Ionic Bonding
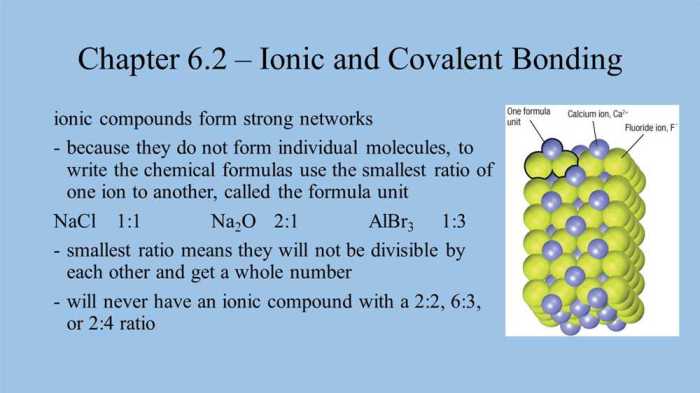
Ionic bonding is a type of chemical bonding that occurs between atoms of metals and nonmetals. In ionic bonding, one atom transfers one or more electrons to another atom. The atom that loses electrons becomes a positively charged ion, and the atom that gains electrons becomes a negatively charged ion.
The oppositely charged ions are attracted to each other by the electrostatic force, forming an ionic bond.
Covalent Bonding, Simulation ionic and covalent bonding answer key
Covalent bonding is a type of chemical bonding that occurs between atoms of nonmetals. In covalent bonding, the atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, forming a covalent bond.
Properties of Ionic and Covalent Compounds
Ionic and covalent compounds have different physical and chemical properties. Ionic compounds are typically solids with high melting points and boiling points. They are also soluble in water. Covalent compounds are typically gases, liquids, or solids with low melting points and boiling points.
They are insoluble in water.
Differences between Ionic and Covalent Bonding
The following table compares the key differences between ionic and covalent bonding:
| Property | Ionic Bonding | Covalent Bonding |
|---|---|---|
| Type of atoms involved | Metals and nonmetals | Nonmetals |
| Electron transfer | One or more electrons are transferred from one atom to another | Electrons are shared between atoms |
| Type of bond formed | Electrostatic attraction between oppositely charged ions | Attraction between shared electrons and nuclei |
| Physical properties | Solids with high melting points and boiling points, soluble in water | Gases, liquids, or solids with low melting points and boiling points, insoluble in water |
Examples of Ionic and Covalent Compounds
Some common examples of ionic compounds include sodium chloride (NaCl), potassium chloride (KCl), and calcium oxide (CaO). Some common examples of covalent compounds include water (H2O), methane (CH4), and carbon dioxide (CO2).
Expert Answers: Simulation Ionic And Covalent Bonding Answer Key
What is the primary distinction between ionic and covalent bonding?
Ionic bonding involves the transfer of electrons, resulting in the formation of charged ions, while covalent bonding entails the sharing of electrons between atoms.
How does the electronegativity of atoms influence the type of bonding formed?
Electronegativity determines the tendency of atoms to attract electrons. When the electronegativity difference between atoms is significant, ionic bonding occurs; when the difference is minimal, covalent bonding is favored.
Can a molecule exhibit both ionic and covalent character?
Yes, molecules can possess partial ionic and covalent character due to the uneven distribution of electrons within the molecule, known as polarity.