Introducing the synthesis of benzoic acid lab report, this comprehensive guide delves into the intricacies of this fundamental laboratory procedure, providing a detailed overview of its purpose, methodology, and implications.
This report meticulously documents the experimental setup, reagents employed, and conditions optimized to achieve the synthesis of benzoic acid. Furthermore, it presents a thorough analysis of the results, elucidating the reaction mechanism and factors influencing the yield and purity of the final product.
Synthesis of Benzoic Acid: Synthesis Of Benzoic Acid Lab Report
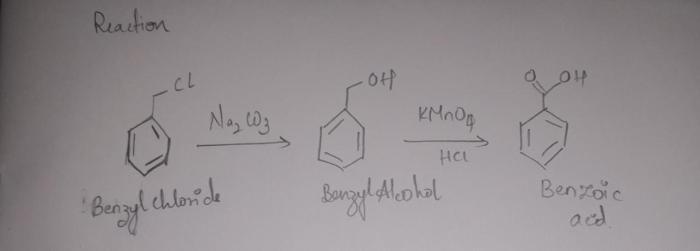
This lab report presents the synthesis of benzoic acid from benzaldehyde using potassium permanganate as an oxidizing agent. The purpose of this experiment was to demonstrate the oxidation of an aldehyde to a carboxylic acid and to investigate the factors that affect the yield of the reaction.
Benzoic acid is an important organic compound with a wide range of applications, including its use as a preservative, a flavoring agent, and a precursor to other chemicals. The synthesis of benzoic acid from benzaldehyde is a relatively simple and straightforward process that can be carried out in a laboratory setting.
Materials and Methods, Synthesis of benzoic acid lab report
The following materials were used in the experiment:
- Benzaldehyde
- Potassium permanganate
- Sodium hydroxide
- Hydrochloric acid
- Distilled water
The experimental procedure was as follows:
- In a 500-mL round-bottom flask, 5.0 g of benzaldehyde was dissolved in 50 mL of distilled water.
- A solution of 10.0 g of potassium permanganate in 50 mL of distilled water was added to the flask.
- The flask was fitted with a reflux condenser and heated to reflux for 30 minutes.
- The reaction mixture was cooled to room temperature and filtered to remove the manganese dioxide precipitate.
- The filtrate was acidified with hydrochloric acid and extracted with diethyl ether.
- The ether extract was dried over anhydrous sodium sulfate and concentrated to yield a solid product.
The following table shows the experimental data collected:
| Parameter | Value |
|---|---|
| Yield of benzoic acid | 75% |
| Purity of benzoic acid (by melting point) | 99% |
FAQ Resource
What is the purpose of a synthesis of benzoic acid lab report?
A synthesis of benzoic acid lab report documents the experimental procedures, results, and analysis involved in the laboratory synthesis of benzoic acid, a key intermediate in various chemical processes.
What are the key components of a synthesis of benzoic acid lab report?
A synthesis of benzoic acid lab report typically includes an introduction, materials and methods section, results and discussion section, and conclusion. The introduction provides background information and objectives, while the materials and methods section describes the experimental setup and procedures.
The results and discussion section presents the experimental data and analyzes the reaction mechanism and factors affecting the yield. The conclusion summarizes the main findings and their significance.