Relative mass and the mole pogil answer key – Embark on a captivating journey into the realm of chemistry as we delve into the concepts of relative mass and the mole, unlocking their significance and practical applications. This comprehensive guide, complete with an answer key, will illuminate the intricacies of these fundamental concepts, empowering you to navigate the complexities of chemical calculations with ease.
Relative Mass and the Mole: Relative Mass And The Mole Pogil Answer Key
Relative mass and the mole are fundamental concepts in chemistry that are used to quantify the amount of substances involved in chemical reactions. Understanding these concepts is essential for understanding the stoichiometry and behavior of chemical substances.
Relative Mass
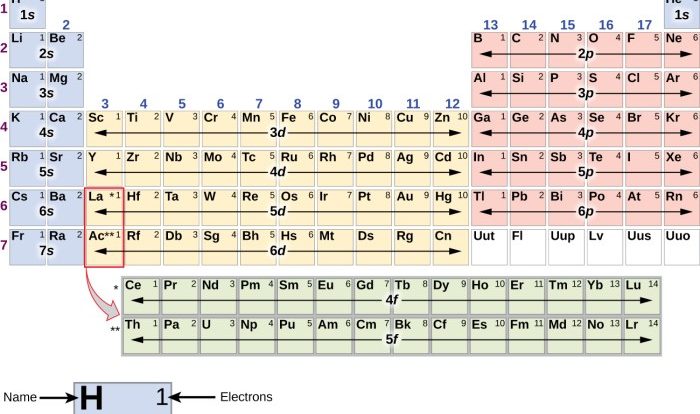
Relative mass is a measure of the mass of an atom or molecule relative to the mass of a carbon-12 atom, which is assigned a relative mass of 12. The relative mass of an element is the weighted average of the relative masses of its isotopes, taking into account their natural abundances.
To calculate the relative mass of an element, we add the relative masses of each isotope multiplied by its abundance as a fraction of the total. For example, chlorine has two stable isotopes, 35Cl and 37Cl, with relative masses of 34.96885 and 36.96590, respectively.
The abundance of 35Cl is 75.77%, and the abundance of 37Cl is 24.23%. The relative mass of chlorine is then:
(34.96885 x 0.7577) + (36.96590 x 0.2423) = 35.45
The Mole
The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains exactly 6.02214076 x 10 23elementary entities. These elementary entities can be atoms, molecules, ions, electrons, or other particles.
Avogadro’s number, 6.02214076 x 10 23, is the number of elementary entities in one mole of a substance. It provides a bridge between the macroscopic and microscopic scales, allowing us to relate the mass of a substance to the number of particles it contains.
To convert between moles and number of particles, we use the following formula:
Number of particles = moles x Avogadro’s number
Molar Mass
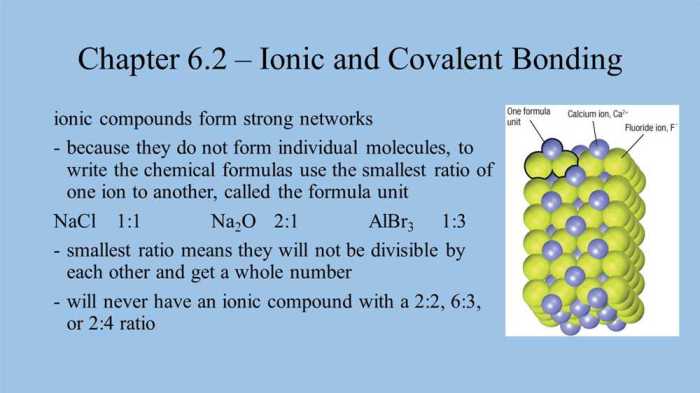
Molar mass is the mass of one mole of a substance. It is calculated by adding the atomic masses of all the atoms in the molecule or formula unit. For example, the molar mass of sodium chloride (NaCl) is:
99 (Na) + 35.45 (Cl) = 58.44 g/mol
Molar mass is a useful concept because it allows us to relate the mass of a substance to the number of moles it contains. This is particularly important in stoichiometry, where we need to know the amount of reactants and products involved in a chemical reaction.
Applications, Relative mass and the mole pogil answer key
Relative mass and the mole concept are widely used in chemistry. They are essential for:
- Calculating the amount of reactants and products in chemical reactions
- Determining the concentration of solutions
- Performing stoichiometric calculations
- Understanding the behavior of gases
- Characterizing materials
Top FAQs
What is the difference between relative mass and molar mass?
Relative mass represents the mass of an atom or molecule relative to a standard, while molar mass is the mass of one mole of a substance.
How do I calculate the number of atoms in a given mass of an element?
Divide the mass by the relative atomic mass and multiply by Avogadro’s number.
What is the significance of Avogadro’s number?
Avogadro’s number defines the number of particles (atoms, molecules, or ions) present in one mole of a substance.